The Diphoterine® solution – emergency washing solution for chemical injuries
Diphoterine® solution is an emergency rinsing solution for splashes of chemical products. Its rapid use in case of contact between the skin or eye and a chemical product is intended to quickly eliminate the residual chemical product on the skin or in the eye. This makes it possible to limit the extent of the burns and lesions caused. Diphoterine® facilitates secondary treatment of the burn injuries by restricting the extent and severity of the lesions.
The knowledge acquired during our research work on the mechanisms of the lesions caused by chemical products on soft tissues have allowed us to develop this technological solution for emergency rinsing of chemical splashes.
Prevention and implementation of first-aid solutions are essential in all situations involving a risk of human skin or eye contact with a chemical product. In case of an incident, Diphotérine® solution allows performance of emergency decontamination.
The European Chemicals Agency (ECHA) has listed more than 75,000 products that are corrosive, irritant or sensitising for the skin, eye or the respiratory tracts. These products are liable to cause serious lesions that may be limited by first-aid use of Diphoterine® solution.
Historically, emergency rinsing with water represented an initial element of crucial progress in decontaminating chemical splashes. Use of water as a first-aid solution has its limits however. Owing to its physical and chemical properties, Diphoterine® solution allows rinsing to be performed more effectively than with water in case of splashing of chemical product.
Diphoterine® solution is an emergency rinsing solution for splashes of chemical products. It is intended for first-aid use on the human body. Its rapid use in case of splashing or contact of the skin or eye with a chemical product makes it possible to limit the extent of the burns and lesions caused.
Diphoterine® solution is a class IIa medical device according to Directive 93/42 EEC. It can be used on injured skin and acts on the chemical product in order to halt the reaction between the chemical product and the tissue and minimise the lesions caused.[1] Diphoterine® solution can be used in complete safety.[2] Diphotérine® solution is guaranteed free of phosphates. Its physical and chemical characteristics allow decontamination in case of splashing of chemical products on the skin or in the eye.
Diphoterine® solution is not a treatment for confirmed chemical lesions. It is definitely an emergency rinsing solution for eye or skin splashes of chemical products.
Diphoterine® solution is an emergency rinsing solution for splashes of chemical products. Its physical and chemical properties allow performance of rinsing if chemicals are splashed on humans.
Four characteristics provide its activity against splashes of chemical products.
Diphoterine® solution is versatile
Its physical and chemical properties make it effective against a wide range of classes of chemical product (acids, alkalis, oxidising agents, reducing agents, alkylating or chelating agents and solvents).
Diphoterine® solution is guaranteed free of phosphates.
Diphoterine® solution is liquid: it therefore allows performance of effective mechanical rinsing of the chemical product on the surface of the skin or the eye.
When a splash occurs, the chemical product is deposited on the surface of the tissue. It does not penetrate instantly and completely into the skin or the eye. Some chemical product therefore remains on the surface. Mechanical elimination by rinsing using a liquid solution allows removal of the product from the surface of the tissue. Consequently, it is no longer capable of reacting with the skin or the eye and of creating a burn. Mechanical rinsing is the major effect of emergency decontamination of splashes of chemical products using Diphoterine® solution.
Irrigation of the contaminated area with a copious amount of liquid Diphoterine® solution also allows dilution of the chemical product. A less concentrated chemical product will be less aggressive to the tissue. This additional physical effect is minor in relation to mechanical rinsing of the chemical product.
Diphoterine® solution will limit penetration of the chemical product inside the tissue.
The chemical products quickly penetrate into the skin or the eye, creating deep-lying lesions.
Diphoterine® solution is a hypertonic solution: it has an osmotic pressure greater than that of the skin and the eye. This makes it possible to limit penetration of the chemical product inside the tissues.
Diphoterine® solution is able to halt the aggressiveness of the chemical product.
Diphoterine solution contains an amphoteric and chelating molecule. The chemically reactive sites of this molecule will be capable of reacting with the corrosive or irritant chemical products, thus preventing their reaction with the biological components of the tissues. Owing to this additional property, rinsing allows more rapid return to an acceptable physiological state.
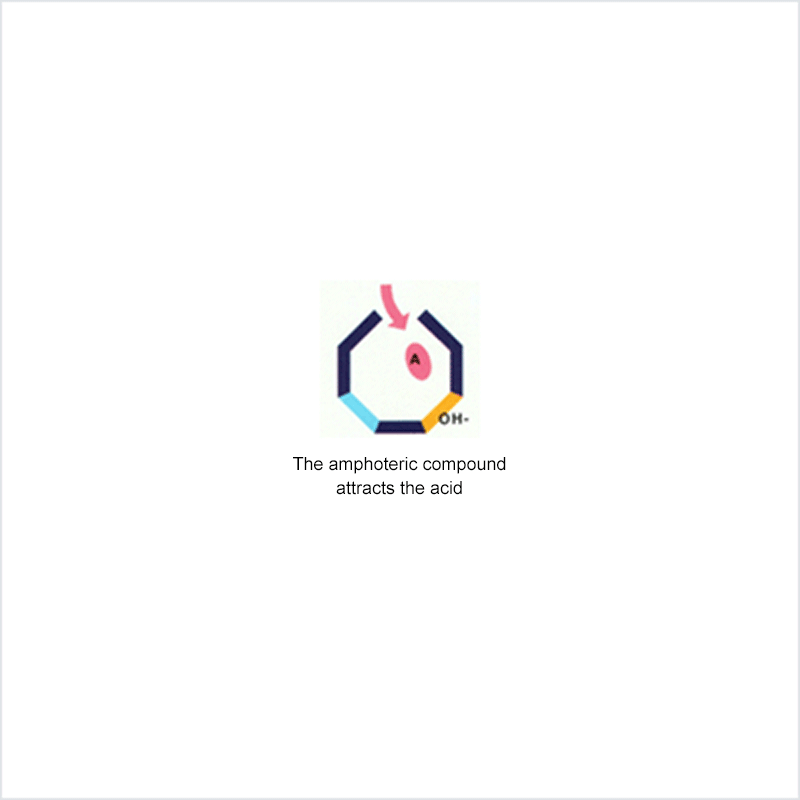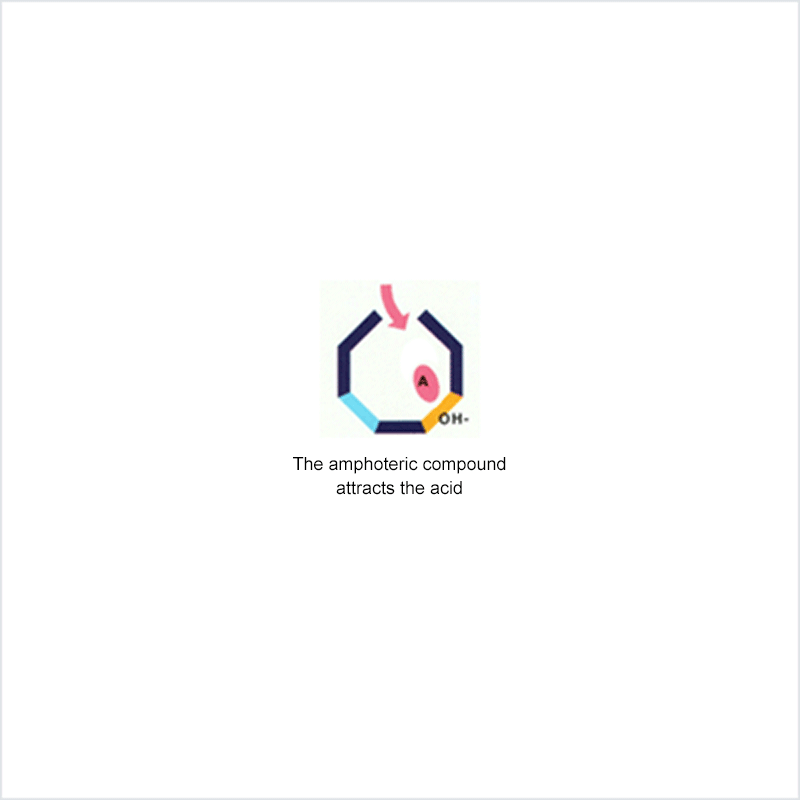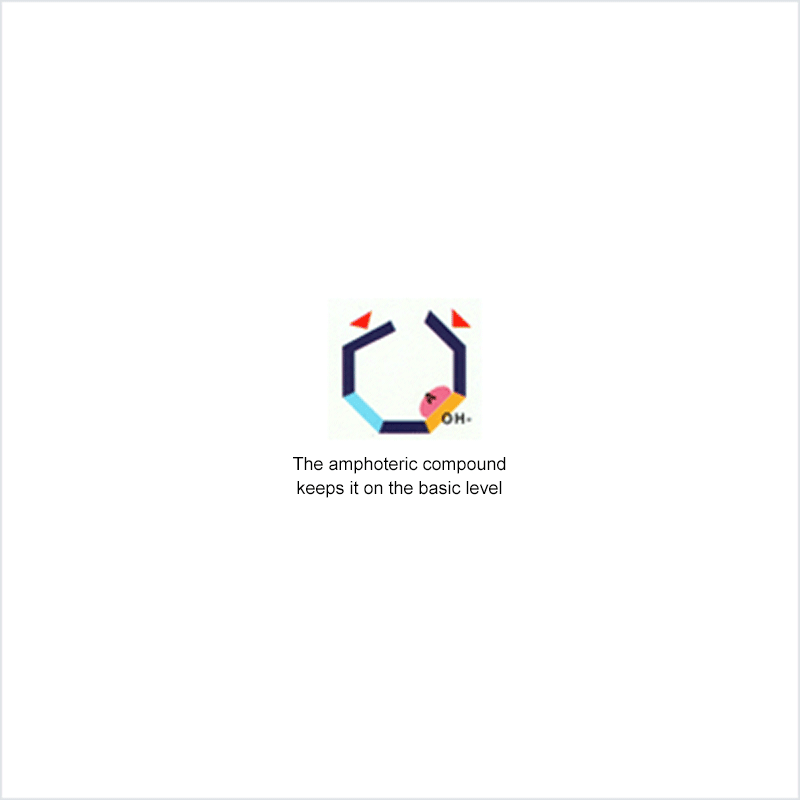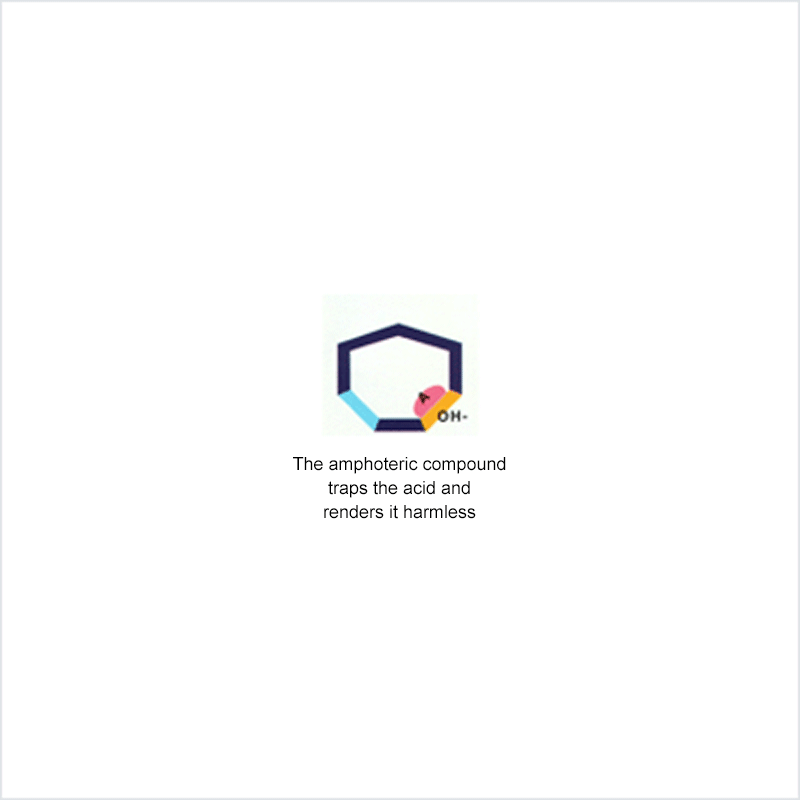

Corrosive and irritant chemical products react with the biological components of the skin or eye. This gives rise to skin or eye lesions or burns, the consequences of which may be very serious.
In the event of contact between a corrosive or irritant chemical product and the skin or eye, it is essential to limit the reactions between these products and the tissues. The chemical product will react with the tissue for as long as it is in contact with the latter and no action halts its aggressiveness.
In order to decontaminate effectively, the contact time and the reactions between the aggressive chemical product and the biological tissue must be restricted. To this end, it is necessary to:
- Eliminate the chemical product present on the surface of the skin or the eye
- Halt penetration of the chemical product inside the tissue and remove the chemical product that has already penetrated
- Limit the corrosive or irritant potential of the chemical product in order to prevent development of lesions.
One must therefore act as quickly as possible and move the chemical product away from its biological targets. Hence, emergency management requires one to:
- Undress the affected person. If the product has splashed on clothing, the latter will be soaked with corrosive or irritant agent which is liable to react if in contact with the skin.
- Perform thorough rinsing, which will allow mechanical removal of the chemical product from the surface of the body and dilute it, thereby making it less aggressive. A potential chemical action of the rinsing solution may also halt the aggressiveness of the chemical product, thereby limiting the lesions that it causes on the tissues.
Quick and thorough washing therefore allows effective decontamination of the tissues which have been brought into contact with a hazardous chemical product.
What are the advantages and limitations of rinsing with water?
“Wash thoroughly with water”. This recommendation for decontamination of chemical splashes is historical and still remains widespread. Nevertheless, washing a splash of chemical product with water has advantages and disadvantages.
It is the first solution offered for emergency decontamination of tissues brought into contact with a chemical product.
Rinsing with water has practical advantages which are often put forward: its availability and versatility. Access to water is indeed generally easy. Furthermore, it allows the vast majority of chemical products to be rinsed off with the same efficacy.
The action of the water on a splash of chemical product is mechanical: the water will rinse off the chemical product and remove it from the surface of the skin or the eye. This mechanical action is the main effect of the rinsing.
Furthermore, rinsing with a copious amount of water will allow dilution of the chemical product. The less the chemical product is concentrated, the less it is aggressive to biological tissues.
Rinsing with water however has a limited action and offers a number of disadvantages.
Rinsing with water encourages penetration of the chemical product inside the tissues. Water in fact has an osmotic pressure less than that of the skin or the eye. Hence, a portion of the water used for rinsing will penetrate inside the tissue, taking a small amount of chemical product with it. The chemical product thus passing through will subsequently be able to react with the tissues, creating lesions and burns. This action is known as the “wash-in effect”[3]
Rinsing with water does not have any effect on the chemical product that has already penetrated into the tissues. Water does not make it possible to eliminate the chemical product that has already passed into the skin or the eye. The quantity of corrosive or irritant product that has penetrated will be able to react with its biological targets and cause chemical lesions. Consequently, if the chemical product has disrupted physiological balance, rinsing with water will not help to restore a physiological state. For instance, an acid that penetrates into the skin will reduce the pH of the skin that it contaminates. Rinsing with water will not allow a return to a normal pH for the skin.
Water does not have any chemical action on the reactivity of the chemical product. Rinsing with water does not allow any reduction in the hazard represented by the chemical product.
The intervention period for rinsing with water is very short in order to obtain an optimum result. In the United States, the norm of the American National Standard Institute (ANSI) recommends beginning rinsing within the 10 seconds following a splash of chemical product into the eye.
Finally, rinsing with water makes it possible to:
- Rinse the chemical product mechanically from the tissue surface
- Dilute it
Experimental studies showed that rinsing with water was not optimum and that it was possible to improve emergency management of a chemical burn, particularly for corrosive and irritant products.[4]
[1] Cavallini, Annals of Burns and Fire disasters 2004, vol XVII-2, 84-87 et Cavallini, European Journal of Anaesthesiology 2004, 21, 389-392
[2] Hall, A. H.; Cavallini, M.; Mathieu, L.; Maibach, H. I. M. Cutaneous and Ocular Toxicology, 2009, 28 (4), 149-156
[3] Moody, R. P.; Maibach, H. I.; Skin decontamination: Importance of the Wash-in effect, Food and Chem. Toxicol. 44 (2006) 1783-1788
[4] Andrews K, Mowlavi A, Milner S The treatment of alkaline burns of the skin by neutralization. Plastic Reconstr Surg 2003 ; 111 : 1918-1921


